Embryology of the Heart
CARDIAC DEVELOPMENT
Abridged from Cardiac Development by Margaret Kirby (2007)
Michael J. Shea, Ph.D.
The development of the human heart from a primitive tube has a very unique and unusual story. The heart is the least embryonic of all the different organs and structures of the human body. This is because it is assembled from so many different cells that come from different areas around it. Heart fields containing future developmental structures are located all around the early embryo. Its final form does not come from its original embryonic structure. For example, the atria are composed from different genetic configurations than the ventricles. This is the story for rest of the heart, as well. The inflow and outflow regions have very different beginnings. The covering of the heart comes from the liver and is very different in origin than the inner lining of the heart. All the other organs and structures of the body are built and assembled from their original embryonic structures except the heart. This gives us a clue about the remarkable ability of the heart to transform itself under a wide variety of influences.
Because the segments of the primitive heart tube have different conception and birth dates, each may come under different gene regulation as the time and spatial patterns of expression for the genes change during early development. These six points are relevant to the sequential formation of the cardiac segments by the fusion of the heart fields.
1. Each of the four segments derived from the heart fields has a different conception and birth date. This means that the starting point of a segment and its completion are all different.
2. Looping, bending and rotation of the heart tube accompany the progressive addition of segments. Looping, bending and rotation cause the heart tube to be turned upside down in early development. Thus the imprint of feeling one’s heart being turned upside down is a normal developmental dynamic.
3. Each side of the early looped, U-shaped heart, has a different number of segments (one on the left and three on the right of the U).
4. A fifth or outlet segment may form outside the heart fields surrounding the U after it bends into an S shape.
5. The segments have different developmental fates.
6. None of the segments are directly equivalent anatomically to a specific chamber of the adult heart as mentioned above; instead, they integrate to form the definitive heart chambers.
Another unique aspect of the human heart is its ability to transdifferentiate heart cells that are already at the end of their fate line and being used nearby to a completely different cell for use in an adjacent area of the heart. The heart must go through a significant amount of bending and folding during its growth that gradually the very inner recesses of the heart have limited access to a source of cells coming from the outside. Consequently cell death is essential in certain areas of the heart, because it provides the space or room for the inner myocardial tissue to be transdifferentiated. The very center of the heart requires death for transformation to occur. That is the deepest imprint of the heart, to die to itself so that it may be completely and thoroughly transformed. It does this naturally.
To unfold this story with any degree of coherence, I am following Margaret Kirby’s excellent work called Cardiac Development (2007). It is here that we find that the human heart lies somewhere between a chicken and a lamb. It is difficult to study the human heart in isolation because of obvious political, ethical and moral constraints. However, enough research has been done with mammalian hearts and the human heart to paint a very compelling picture of the most unembryonic of human structures. And therefore it is unique.
Organogenesis
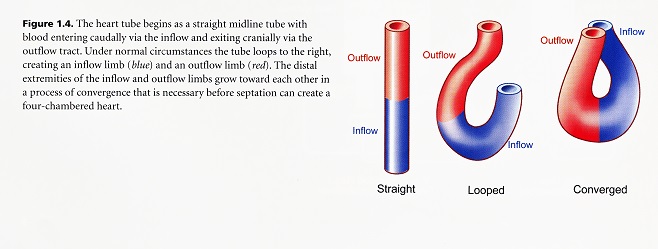
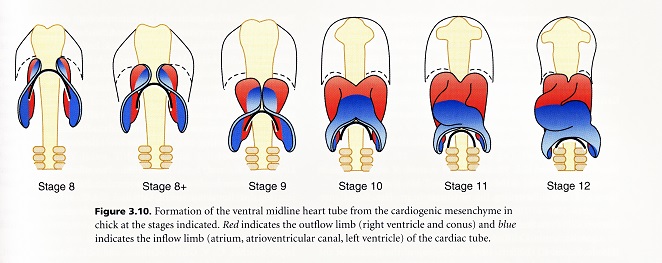
The heart is the first organ to arise and function during embryonic development. Its pattern from fish to humans is somewhat similar. A bilateral tube must join in the ventral midline to form a simple tubular heart; looping and rotation to the right side of the midline; chamber specification and formation and finally, the development of specialized nervous conduction tissue, coronary circulation, innervation by the autonomic nervous system, and finally the maturation of its valves.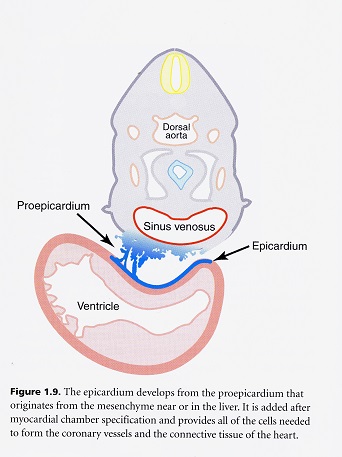
Vascular Development
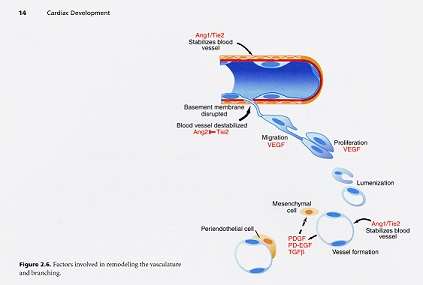
The blood is an organ unto itself containing specialized connective tissue cells and arises before the heart does centrally. The vascular system comes about from two major processes called vasculogenesis and angiogenesis. Blood vessels are created via vasculogenesis and from the blood vessels come the blood cells and consequently, angiogenesis. Once a flow of fluid begins, the primary capillary plexus is remodeled into a hierarchical system of large to small blood vessels and regression of existing channels.
The development of the vascular system begins outside the central body of the embryo in the yolk sac. These blood cells coming from the yolk sac begin producing other blood cells that form and extraembryonic capillary network that much later in development will become the placenta. Vascular development is initiated in the embryo itself soon after it begins in the yolk sac. The repatterning and remodeling of organized blood vessels to an adult configuration depends on a great many biomechanical, biodynamic and genetic queues.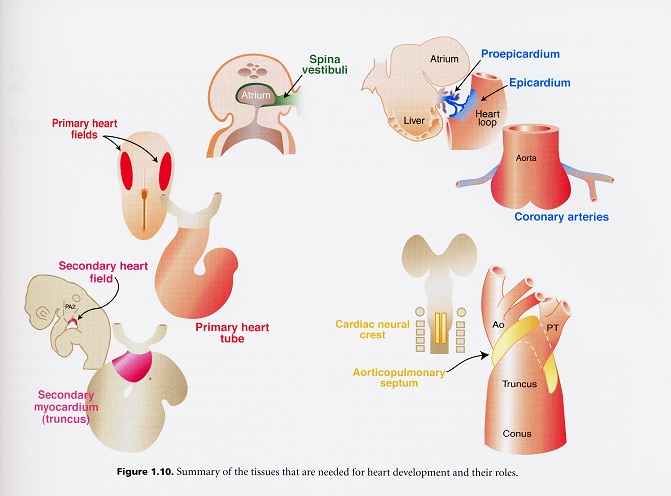
Little is really known about the initial vascular patterns in the embryo. It is thought that the neural tube acts as the initial vascular patterning center. The first recognizable vessels in the embryo are the bilaterally paired dorsal aortae. Some organs, including the heart, acquire their early vessels by vasculogenesis, followed by angiogenesis within the organ, although other organs do not use this combination and will form either with one or the other.
The sinus venosous is the major collecting point for all the blood returning to the heart. When it is formed originally, it is bilaterally symmetrical, but as with the rest of the heart, it quickly shows asymmetrical patterning and becomes largely incorporated into the right atrium. In our early mammalian ancestry, the coelomic sacs originally performed this function of collecting point for all the blood returning to the heart.
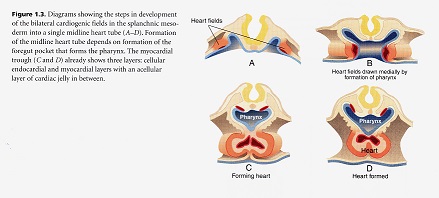
Heart Fields and Heart Tube
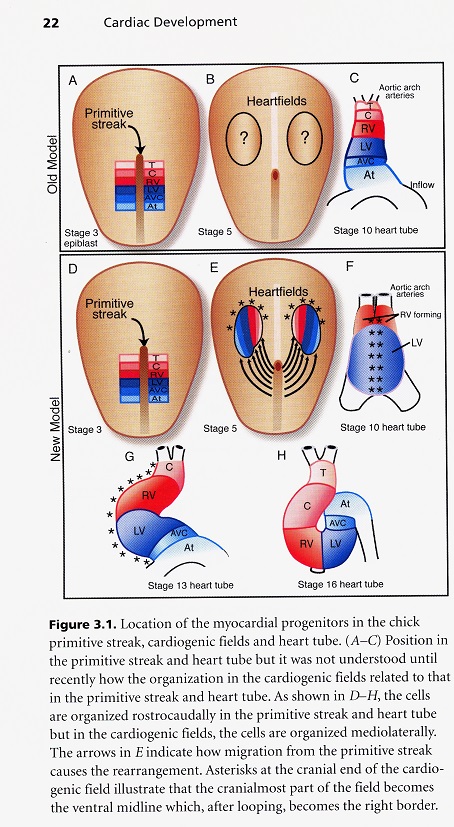
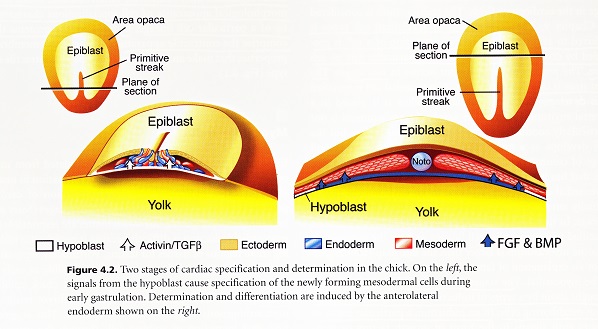
All vertebrates go through a type of development in which the original pleuripotential field of stem cells becomes established first and then subdivided into organ fields as development proceeds. The heart field of cells is established in a canalization zone of spiraling fluids in the amniotic sac of the embryo. With the emergence of the primitive streak which opens the space between the ectoderm and endoderm, the heart fields are established in the mesodermal germ layer. These fields are then subdivided spatially and molecularly into chamber forming regions. The way in which these fields are brought together and how the chambers are established are exceedingly complex. The myocardial stem cells are generated from epiblast cells that ingress through the primitive streak during the third stage of dynamic morphology when the mesoderm is formed. These early cardiac cells are concentrated along the primitive strength and around the primitive node. These original cells form both the endocardium (inner lining) and myocardium (middle layer of the heart) and an addition to non-cardiac populations, which are the dorsal mesocardium and the parietal pericardium. The cells in the primitive streak are located in the same rostrocaudal sequence as that found in the heart which is referred to as co-alignment. This means that the most rostral cells in the streak form the most rostral region of the heart tube, and the cells most caudal in the streak form the most caudal region of the heart too.
After their ingression through the primitive streak the cardiogenic mesodermal cells rapidly move laterally and cranially until they take their position in the lateral plate mesoderm. Once this heart field is formed bilaterally, it then becomes quickly subdivided as can be seen by the differential expression of the genes in the field. The subdivisions represent regions of what will become the inflow and outflow portions of the tube. The idea that this is a tube is a misconception however, because the dorsal myocardial seam adjacent to the foregut has not closed. The atrioventricular canal and atria are added from a region that has been called the secondary heart field, but is likely just a subdivision of the cardiogenic mesoderm. It must be remembered that pharyngeal arches number one and two have substantial mesodermal cores while the mesodermal contribution to arches three, four and six is much less. Studies have shown that the pharyngeal mesoderm progressively moves into the lengthening heart tube. It is interesting to ponder the importance of the pharynx and its connection into the heart. This means that heart tube and foregut formation are interrelated. The foregut elongates as a result of tension applied at the anterior intestinal portal. This tension arises from the regression of the primitive streak and elongation of the notochord. The new model of heart development shows that the heart ascending outflow precursor cells form from the anterior heart field. The outflow and inflow precursors invert their position to give rise to their prospective segments. This inversion is accomplished by a 120 degree rotation of the heart field along with the coelomic cavity in the same direction with the formation of the foregut. The heart fields then fuse anteriorly at the midline. The foregut pocket is a lateral spreading of the endoderm in the cranial portion of the embryo. This model accounts for how the caudal-cranial polarity found in the primitive streak and heart tube is maintained by the 120 degree lateral rotation of the cardiogenic plate found in early human heart development.
The Frank-Starling Relationship
A common model for heart development is the lamb. This is because the fetal and newborn heart of a lamb is similar in size to that of a human. Several animal studies have shown that in normal human neonates and infants, there is reduction in contractility and systolic function with growth. This reduced contractility appears greater than can be accounted for by the increased load and wall stress in the heart that occurs after birth. The Frank-Starling relationship or Starling’s Law of the Heart is characterized by an increase in systolic pressure with increasing ventricular volume. A key observation in these studies is that the fetal heart appears to be operating close to the top of the Starling relationship, thus there is less reserve in response to circulatory stress. Normally, in the fetal environment, a limited Starling response would not be a problem. However, after birth, the rapid and immediate increase in left ventricular volume may expend the immature heart’s Frank-Starling reserve, so that there is even less ability as a human baby to respond to circulatory stress.
The Poles of the Heart
The poles of the heart are connected with the venous inflow and arterial outflow. Many heart defects are the result of incorrect connections at the venous or arterial pole. During embryonic development, the poles provide an important entryway for new cells to be added to the heart. They also provide access to the endocardial and cardiac jelly layers located beneath the myocardium. This is especially important in the outflow track where little mesenchyme is available from any other source.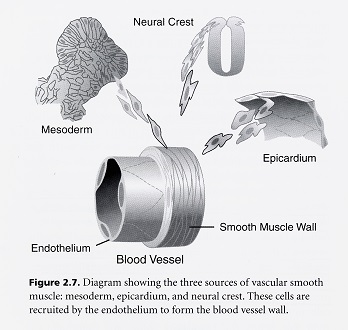
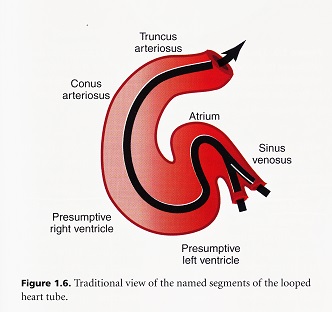
Development of the initial heart tube is from the cardiogenic fields and all the precursors of the conus, right ventricle, left ventricle, atrioventricular canal, and atrial appendages are represented in the early heart tube before the dorsal mesocardium completely disappears. As it disappears, this releases the heart from the ventral pharynx. It leaves only the truncus to be added to the arterial pole and the body of the atria and atrial septa to be added to the venous pole. The conus is derived from the mesodermal cores of pharyngeal arches 1, 2 and 3.
The vestibular spine is a structure originating in the splanchnic mesoderm, ventral to the foregut. It is thought that it remodels into the primary atrial septum. The derivatives and position of the spine are important because variability in the connection of the atrial chamber to the body of the embryo, via the dorsal mesocardium and vestibular spine influence the atrial relationship to the extracardiac midline mesenchyme. This connection the midline is critical, because it encloses the pulmonary pit which is the entry point of the pulmonary vein into the heart. Any variation in this connection has the potential for abnormal positioning of the pulmonary vein and consequently abnormal pulmonary venous return. Once again it is shown that the prepharyngeal mesoderm has an important relationship with the myocardium and that the other mesenchyme in the pharynx is not on the same tissue plane as the myocardium. It is here in the poles of the heart that spiraling motion is constantly seen as cells move into place.
Looping
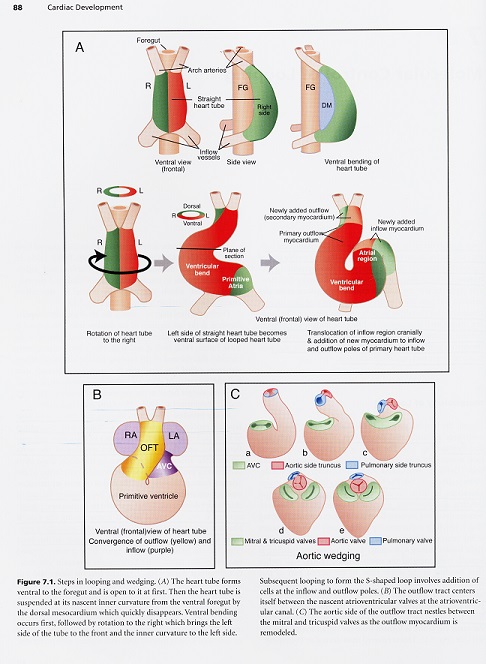
Cardiac looping is the first visible sign of right-left asymmetry during embryonic development. The heart always loops to the right. The looping process itself is important to bring the initially sequentially ordered regions of the heart tube with serial circulation into the correct confirmation for chamber specification and septation to create two parallel circulatory systems. Looping involves at least four separate processes. The first is bending into a sea shaped loop. The C-shaped loop rotates to the right and secondly the heart tube now elongates through peristaltic movement to form an S-shaped loop. This S-shape creates convergence of the inflow and outflow poles such that both the openings seem to be at the top. Finally, there is rotation and wedging of the aorta between the atrioventricular valves.
An important feature of human heart development is that the initial tube is formed along the ventral pharynx, where there is ample craniocaudal space for a relatively long tube to form. It is important to note that the direction of head turning in the embryo is controlled by the asymmetry of heart looping. The first sign of the head turning occurs in conjunction with heart looping. This suggests a multistage process in which a right-left axis is determined during the third stage of dynamic morphology. The second process is the coordination then of a right-left head axis during the formation of the foregut pocket when the cardiac tube is formed.
Looping begins by ventral bending and rotation to the right. After this, elongation occurs as cells are added to the ends of the tube because the attachment of the tube to the ventral foregut by the dorsal mesocardium disappears as looping begins. This detachment seals the dorsal midline seam of the tube, making the sides of the tube inaccessible and leaving only the ends of the tube attached and open for cells to come in. Wedging is the moving of the aorta behind or caudal to the pulmonary trunk. It occurs during septation and is dependent on retraction and rotation of the truncal myocardium by about 45 degrees. Compaction and rotation of the truncal myocardium are accompanied by cell death, suggesting that cell death is one mechanism for shortening.
Typically, heart formation is associated with the primitive streak and the establishment of a left-right body axis. The connection with the craniocaudal axis is via the primitive node or Henson’s node, which lies at the cranial end of the primitive streak. It is the node that generates the tissue of the notochord that forms the midline. In the adult body, primitive node goes as high as the third cervical vertebra before it recedes in the embryo back to the second sacral segment. However, it is now known that there is an asymmetrical expression of genes at the primitive node. The role of the primitive streak caudally and the notochord cranially takes on critical importance in the asymmetry of gene expression and the growth and differentiation of the heart. The right and left atria of the heart are established through the symmetrical heart fields, but most of the rest of the heart, including the ventricles are constructed from asymmetrical locations in the developing embryo.
Chamber Development and Septation
Due to asymmetrical gene expression, the craniocaudal axis is the only axis that maintains its symmetry as the heart develops. The other axes are remodeled as this semiautonomous heart tube forms. As it forms, new axes are established in order to manage the process of looping just described as well as the development of the four chambers and their septation. Septation refers to the connective tissue dividers between the two atria and two ventricles. The development of four specific chambers occurs through discrete gene transcription programs. These programs govern myocardial identity during the stages of specialization in the forming heart. These genetic programs are established based on the polarity of the craniocaudal and dorsoventral axes of the heart tube.
When both the inflow and outflow ends of the heart tube form, they are built with different populations of surrounding cells. After the c-shaped looping, the deepest convexity of the looped heart separates these inflow and outflow poles of the tube. This bend in the tube gives an approximate position for where the ventricular septum will form to separate the right and left ventricles. The chambers are gradually sculpted from the addition of cells at the inflow and outflow poles.
The primary atrial septum is cardiac muscle that is incorporated form the splanchnic mesoderm near the venous pole. As the septum extends from the dorsal-atrial wall, its leading edge is covered by mesenchyme. This primary atrial septum is augmented by a secondary septum which is constructed from a fold of the atrial myocardial wall during fetal life. This completes the process of atrial septation. By having a primary and secondary atrial septum, a flap valve is created that allows the passage of blood between the two atria since pulmonary circulation has not yet begun in the embryo or fetus. This flap valve, however, must close permanently during the first year after birth in order to initiate proper pulmonary circulation. The left and right atria are derived as mentioned from the left and right cardiogenic fields respectively and retain the left-right body axis specification. Each of the ventricles however is composed of myocardial cells from both the left and right cariogenic fields. Consequently, the left-right polarity has to be re-established in the ventricular section of the heart tube.
Both the right and left ventricles are formed by the ballooning of the outer curvature of the myocardium. This occurs concurrently with the formation of the trabeculae which are myocardial diverticula extending from the compact outer wall of the myocardium into the cardiac jelly toward the lumen. The formation of the trabeculae is the first sign of the differentiation of the two ventricles. Growth is followed by increasing trabeculae which gives the ventricular lumen a spongy appearance. The functional significance of trabeculae in early heart development is unknown. In human embryos at five weeks, a muscular septum is identified between the trabecular portions of both ventricles.
The last part of the ventricular septum to form is the membranous septum which forms from cushion tissue that fills in the opening left between the crest of the ventricular muscular septum and the atrioventricular mesenchymal septum. As the membranous portion of the septum develops, it also forms the primordium of the tricuspid valve. During development, it is detached from the interventricular septum at about the fifth month of fetal development. The muscular ventricular septum is divided into regions designated as the inlet portion near the atrioventricular septum and valves while the portion nearest the ventricular outflow is designated the outlet septum. The bundle of His is directly related to the AV bundle (atrioventricular) including the node. The major function of the AV node is to provide for a delay in an electrical impulse from the atrium to the ventricles.
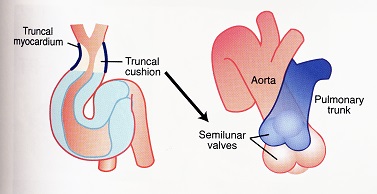
The conus and truncus connect the right ventricle with the aortic sac. The aortic sac is the collecting point of all the blood at the arterial pole of the heart. It is not covered by myocardium and is thus not part of the heart. The conus undergoes dramatic remodeling to become incorporated as part of the connection between the aortic vestibule that connects the body of the right ventricle with the pulmonary trunk and the left ventricle with the aorta. The basic pattern of development is that the myocardium spirals into the truncus. This is the same pattern in which the distal cushions of the ventricles form that ultimately establishes the direction of the spiraling outflow septum. The aortic sac branches into a series of aortic arteries that are suspended from ventral to dorsal in the pharyngeal arches to carry blood to the dorsal aorta. The aortic sac is divided into the pulmonary trunk and aorta by the aorticopulmonary septum. As outflow septation proceeds the developing aorta rotates posterior to the pulmonary trunk and shortens to about 25% of its original length.
The myocardium is the last to be added to the heart tube while the endocardium is derived from both cardiogenic mesenchyme and noncardiogenic regions of the lateral plate mesoderm and head mesenchyme. The mesenchyme in the cardiac jelly of the truncus and conus is contributed to by pharyngeal mesenchyme and ectomesenchymal derivatives of the neural crest. A mesenchymal septum grows from the roof of the aortic sac between the origins of arch arteries 4 and 6. This divides the aortic sac into the base of the aorta and pulmonary trunk before entering the outflow track. As the septum grows into the outflow track it spirals 180 degrees. During septation, the outflow myocardium is remodeled by a combination of incorporation into the right and left ventricles and cell death. This results in the wedging of the aorta behind the pulmonary trunk.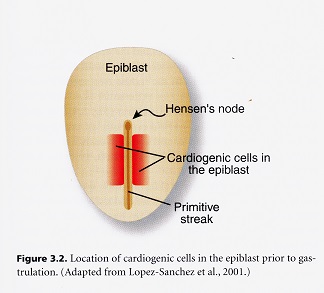
The manner in which the heart fields’ fuse establishes the craniocaudal axis as it was in the primitive streak. This axis is particularly important in establishing the relationship of the primitive atrium, atrioventricular canal, and left and right ventricles which will form from caudal to cranial as the heart tube forms. Thus right and left in the body axis become dorsal and ventral in the heart tube. The dorsal midline seam forms from the most caudal region of the cardiogenic field while the ventral midline seam forms from the most cranial cardiogenic field. The dorsal and ventral midlines of the heart tube are thus formed from the cranial and caudal portions of the cardiogenic fields respectively. Once looping has occurred, they become the outer and inner curvatures respectively. The ventricular chambers expand from the outer curvature. As mentioned, the atria maintain right and left sided identity with the right and left sides of the body. The ventricles, however, are free to re-establish an endogenous understanding of left and right using mixed parts of both cardiogenic fields. The atria must be faithful to the left and right sides of the body so that the asymmetric venous systemic and pulmonary connections are made correctly. It appears that the formation of definitive chamber myocardium requires a regional myogenic specialization that ultimately leads to unique contractile, excitation and cytoskeletal properties unique to each chamber.
The endocardium may be a source of information important to establish the identity of chamber myocardium. Trabeculae that form on the inner surface of the ventricular myocardium are more differentiated and less proliferative than the compact outer layer of myocardium. Cardiac jelly is secreted by the myocardium and is important for propagation, integration, regionalization, and/or stabilization of endocardial signals. By directional signaling between the endothelial and mesenchymal compartments are essential for proper angiogenesis and heart development.
Endocardium
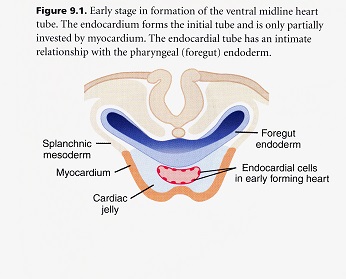
The endocardium develops from endothelial cells that form bilaterally paired tubes by the process of vasculogenesis. These bilateral tubes fuse in the midline to form a singular tubular cardiac intravascular compartment. The midline endothelial tube lies beneath the foregut endoderm and is covered ventrally by the splanchnic mesoderm that is open toward the ventral pharynx in the shape of a W or U until the two sides fuse dorsally to become a complete myocardial layer of the tubular heart. An important feature of the endocardium is that it gives rise to all of the cushion mesenchyme in the atrioventricular canal and proximal outflow track by a process of epithelial-to-mesenchymal transformation. This cushion tissue functions as a one-way valve during contraction of the tubular heart and is critical for formation of the atrioventricular valves. The mesenchyme that gives rise to the semilunar valves in the outflow track is from a mixture of what is called cushion mesenchyme derived from endocardial epithelial-mesenchymal transformation, neural crest and pharyngeal mesenchyme.
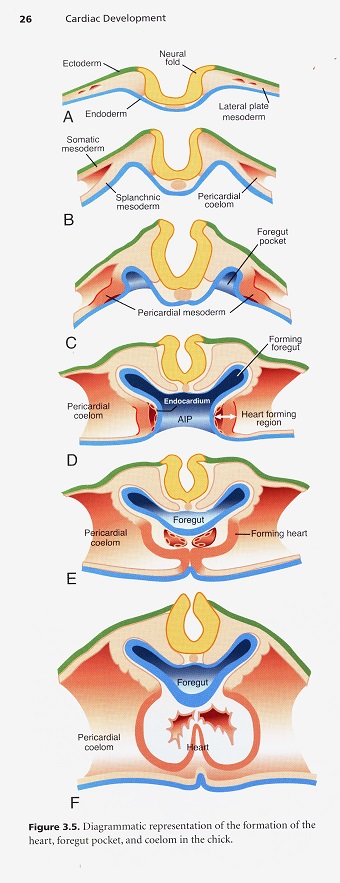
All intraembryonic mesoderm contains endothelial precursor cells that are invasive and have the ability to adapt to any region where they are placed. These characteristics distinguish them from all other embryonic mesenchymal cell types. The cardiac cushions are the primordia of the valves and septa of the adult heart. They are also critical in maintaining anterograde blood flow in the embryonic heart. Embryos that fail to develop cushions die at the onset of chamber septation. Most of the distal outflow cushion mesenchyme is produced by cells that migrate into the outflow cushions from the pharynx.
AV valves include the valve leaflets and a connecting tensile apparatus which consists of both the chordae tendineae and their myotendenous junctions with the papillary muscles. The function of the tensile apparatus is to maintain the valves closed during ventricular systole. The leaflets and tensile apparatus are formed mostly by a process of delamination of the inner layers of the inlet zone of the right ventricle. The atrioventricular endocardial cushions fuse and condense into distinct mitral and tricuspid valve primordium which remodel to form the leaflets and supporting structures. The leaflets are comprised of three recognized layers in both the atrial ventricular and semilunar valves. The layers and the atrioventricular valve leaflets are called the atrialis, the spongiosa, and the fibrosa from the atrial to the ventricular sides. The same three layers are recognized in the semilunar valve leaflets. The septal leaflets or cusps of the mitral and tricuspid valves develop from the superior (dorsal) and inferior (ventral) atrioventricular cushions. The leaflets delaminate from the ventricular septal wall. The leaflets are excavated from the myocardial wall by myocardial cell death, which frees the fibrous leaflets.
Elongation and remodeling of the primordia into mature valve structures is associated with regionalized cell proliferation of valve progenitor populations. The maturation of the valve primordia is accompanied by decreased proliferation.
Epicardium
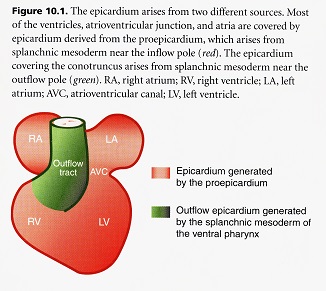
During its early existence, the wall of the tubular heart is composed of only two cellular layers, the myocardium and endocardium. These cellular layers are separated by an acellular cardiac jelly. The third cellular layer, the epicardium, develops mostly from the extracardiac population of cells called the proepicardium migrating from the hepatic plexus or future liver. The proepicardium spreads over the myocardium surface during looping which is well after the heart begins to function. The proepicardium originates from the splanchnic mesoderm caudal to the attachment of the spinous venosus. Protrusions of cells form near the sinus venosus. The protrusions elongate to form finger-like processes that touch the dorsal wall of the ventricle. This initial contact invests all of the myocardium of the atria, atrioventricular canal and ventricles in a single layer of epithelial cells called the epicardium.
A completely separate source of cells derived from the splanchnic mesoderm of the ventral pharynx near the attachment of the outflow pole forms the epicardium of the outflow track. The epicardium covering the atrioventricular canal and ventricles generates an extensive population of cells by epithelial-mesenchymal transformation. These cells form the coronary vasculature (endothelium and smooth muscle), blood progenitors, and fibroblasts (connective and cushion tissue). The first areas to be covered by epicardium are the atrioventricular and conoventricular grooves with the ventricular surface being quickly filled in. The atria and outflow tract are the last to be covered. Some of the newly formed epicardial cells undergo epithelial-to-mesenchymal transformation. By this process of delamination, the epicardial monolayer generates a rich variety of cells that populate the developing heart. The epicardium is required for normal growth and maturation of the myocardium. If the epicardium fails to grow over the surface of the myocardium through genetic defects or mechanical prevention, the embryo dies because of cardiac failure. The interaction of myocardium and epicardium is necessary for myocardial growth. Without epicardium, the myocardium is thin and the ventricular septum fails to form properly.
The formation of a functional coronary vasculature is essential for normal heart development. The coronary vasculature develops from an endothelial plexus that envelops the heart in the same temporospatial pattern as epicardium, but with a delay caused by the transformation and induction steps needed to generate endothelial cells. The plexus first assembles in the atrioventricular and ventricular outflow tract junctions followed by the ventricles and atria. The outflow tract is the last to develop a bulbar plexus. Coronary circulation begins as venous sinusoids that connect with trabecular channels. These trabecular channels connect with arterial vessels that produce a closed coronary circulatory system that is not completed until after septation is completed. The last step in coronary vascular development is ingrowth of multiple small vascular channels into the base of the aorta. The small vascular channels coalesce into a single coronary system of stems in the left and right coronary sinuses.
Neural Crest, Great Arteries and Outflow Septation
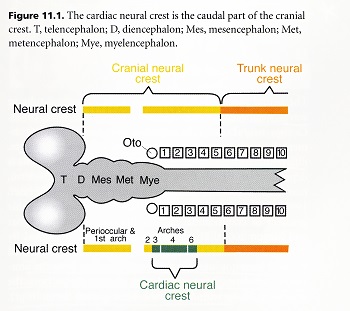
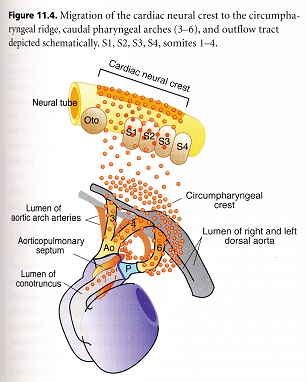
The neural crest is a population of multipotential cells that delaminate from the dorsal neural tube and migrate widely throughout the body. Based on the cells’ axial level of origin form the neural tube, the neural crest is divided into cranial and trunk regions. Both cranial and trunk neural crest cells give rise to the peripheral nervous system and melanocytes. However, only cranial neural crest has the potential to give rise to the “ectomesenchymal” cells. Ectomesenchymal cells take part in development of a wide variety of structures in the head, neck, and heart. Because of the importance of neural crest-derived ectomesenchyme in the heart development, a sub region of the cranial neural crest has been called “cardiac neural crest.” Cardiac neural crest cells also support development and final patterning of the great arteries of the thorax. Neural crest cells are essential for modulating signaling in the caudal pharynx, which is important for normal development of the arterial pole of the heart. A subpopulation of cardiac neural crest cells migrates from the caudal pharynx into the cardiac outflow tract to form the aorticopulmonary septum, which divides the common arterial blood flow into pulmonary and systemic streams in animals with lungs.
Crest cells are divided broadly into cranial and trunk based on their ability to give rise to ectomesenchyme: only cranial neural crest has this capacity. Cranial neural crest originates from mid-diencephalon to somite 5 and trunk crest extends from somite 5 to the caudal tip of the neural tube. Cardiac crest is a subdivision of the cranial crest. It originates in the neural folds spanning from the middle of the otic placode to the caudal border of somite 3 which corresponds with rhombomeres 6, 7 and 8. The cardiac crest represents a transitional domain between the cranial and trunk regions of crests because it shares some properties common to both regions. It lacks the ability for regeneration like the trunk region of the crest. Some of the pre-otic regions of crest are capable of remarkable feats of regeneration from the more ventral neural tube.
The cardiac neural crest provides all of the parasympathetic innervation to the heart. In addition, ectomesenchymal cells from the cardiac crest form the smooth muscle tunics of the great arteries and the connective tissue of glands in the neck – thymus, thyroid, and parathyroids. It also forms the aorticopulmonary septum that divides the outflow tract into systemic and pulmonary outlets. Trunk neural crest provides sympathetic innervation but does not contribute to the structural development of the heart.
The neural crest cells migrate away from the neural tube in waves beginning around the time the neural tube closes. Cardiac neural crest cells require a wide variety of environmental signals in order to be 1. specified, 2. migrate, 3. proliferate, 4. differentiate, and 5. survive. Specification of neural crest cells is dependent on signals from either non-neural ectoderm, non-axial mesoderm or both. Recent experiments suggest that neural crest specification may involve multiple successive inductions.
Cardiac crest cells first migrate into the CIRCUMPHARYNGEAL RIDGE, where they pause while pharyngeal arches 3, 4 and 6 are formed. As each arch forms, the crest cells migrate in and surround the nascent aortic arch arteries that form from strands of endothelial cells initially attached to the pharyngeal endoderm. Non-cardiac cranial neural crest cells populate arches 1 and 2, where they mostly develop into skeletal elements in the arches. In contrast, the caudal arches (3, 4, and 6), which are populated by cardiac neural crest cells, are largely devoted to glandular and vascular development.
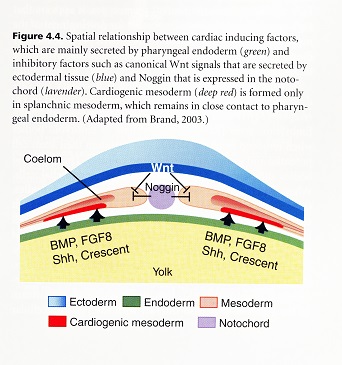
The parathyroid and thymus glands are generated from endoderm in the third pharyngeal pouch, and their development is dependent on an interaction of the neural crest and endoderm. The neural crest cells in arches 3 and 4 induce endoderm to form the glands. Communication is two way because neural crest patterning is influenced by signals form the endoderm.
Cardiac crest cells play an important role in repatterning of the initially bilaterally symmetrical arteries into the asymmetric great arteries. The repatterning steps that convert the bilaterally symmetrical aortic arch arteries to the great arteries are also dependent on the presence of neural crest cells in the caudal pharynx.
After the cardiac neural crest cells migrate into arches 3, 4, and 6, they proliferate. A subset of the cells continues migrating into the cardiac outflow cushions. The cushions themselves are ridges of mesenchyme that spiral into the outflow tract. The mesenchyme that populates the cushions migrates into the outflow tract from the pharynx. The neural crest cells follow these preformed cushions and collect as condensed cells in two columns or prongs centered in the distal or truncal outflow cushions. The prongs are connected distally by a shelf of mesenchymal cells that protrude into the dorsal wall of the aortic sac. It is located between the origins of the fourth and sixth pairs of arch arteries and is thus correctly placed to separate the systemic from the pulmonary outflow (the fourth arch will become the arch of the aorta and the sixth arch will become the ductus arteriosus). The prongs end abruptly when they reach the proximal or conal portion of the outflow tract. The prongs of condensed mesenchyme connected by the shelf of tissue in the aortic sac are called the aorticopulmonary septation complex. This complex does not extend into the conus, where neural crest cells are dispersed subendocardially in no identifiable pattern.
Outflow septation occurs by three different mechanisms based on this initial configuration of neural crest cells. The shelf protruding into the dorsal wall of the aortic sac provides the initial division between the systemic and pulmonary blood streams. The shelf elongates into the distal outflow tract at the expense of the prongs. Because the prongs themselves spiral, the elongating shelf spirals. After the prongs are used in building the distal portion of the outflow septum in the aortic sac and truncus, the proximal outflow septum in the conus closes zipper like from distal to proximal toward the ventricles. This appears to occur as the proximal (conal) cushions are myocardialized. MYOCARDIALIZATION takes place when invading myocardial cells cause the cushions to bulge in the lumen. The bulging cushions meet in the middle of the lumen. When they touch, the endocardium covering the cushions breaks down, allowing mixing of the underlying mesenchyme and myocardium and this brings about the fusion of the opposing cushions to form a septum. Because the neural crest cells were sub endocardial prior to the fusion, they now appear as a seam where the two cushions fused. The process of myocardialization is induced by unidentified factors produced by the non-myocardial component of the outflow tract and can only be induced in the outflow myocardium during the period of outflow septation.
Shear stress may play a role in remodeling the cushions to valve leaflets because interfering with blood flow alters endothelial morphology and causes malformation of the aortic valve leaflets.
In the pharynx, the aortic arch arteries are remodeled. In the chick the right and left aortic arch 3 become the right and left brachiocephalic arteries. In mouse and human, aortic arch arteries 3 form the common carotids. In the chick, the right fourth aortic arch artery becomes the aorta, while the left fourth aortic arch artery disappears. In mouse and human, the right fourth aortic arch artery becomes the base of the subclavian artery and the left fourth aortic arch artery becomes the arch of the aorta. In the chick, the sixth arch arteries become bilateral ductus arteriosus. In mouse and human, the left sixth aortic arch artery forms the ductus arteriosus and the right sixth aortic arch artery disappears.
After this remodeling is complete, the neural crest cells that have surrounded the arteries from the time they migrated into the pharyngeal arches now form a tunica media composed of vascular smooth muscle cells around each of the remodeled arteries. However, these sheaths do not abut the myocardium. The most proximal walls of the aorta and pulmonary trunks are not covered by the neural crest-derived tunics, leaving a gap. This gap of non-neural crest-derived smooth muscle cells is provided by cells from the secondary heart field that have interposed between the neural crest covering the artery that remains after repatterning and the outflow myocardium.
Development of the Conduction System
It is important that the tubular heart and the four-chambered heart beat in a coordinated fashion to propel blood from the inflow to the outflow. In a straight heart tube, because the myocardium is electrically coupled, the solution is relatively straightforward. But, coordinated contractions are necessarily different in a tube versus a four-chambered heart. Thus, development of the pacemaking and conduction system represents one of the most intriguing processes in heart development. Coordinated cardiac contractions are regulated by impulses that originate at the sinoatrial (SA) NODE, located at the junction of the superior vena cava and right atrium. Impulses generated at this node spread through the atrial chamber myocardium, to initiate contraction of the atria. The impulse is also spread to the atrioventricular (AV) NODE, located in the base of the atrial septum at the junction of the atria and ventricles, and is the only conducting route from the atria to the ventricles. The main function of the AV node is to slow the impulse from the atrial to the ventricular myocardium. Transmission of an impulse directly from the atrial myocardium to the ventricular myocardium is prevented by a nonconducting band of connective tissue at the atrioventricular junction called the ANNULUS FIBROSIS. The impulse is delayed by the atrioventricular node to allow time for atrial contraction before ventricular excitation. After the slight delay the impulse is propagated rapidly via the atrioventricular (His) bundle to the BUNDLE BRANCHES located in the top of the ventricular septum. The bundle branches divide on either side of the ventricular septum into branches that terminate in a highly ramified network of PURKINJE FIBERS that will activate both ventricles simultaneously, beginning at the APEX. The impulse is transferred from the Purkinje network into the working ventricular myocardium.
Contraction of the heart proceeds from inflow to outflow in a straight heart tube that needs no paths of excitation. In the mature 3 or 4 chambered heart, blood must be efficiently propelled through the systemic and pulmonary circulations with a coordinated ventricular contraction that begins at the apex of the heart and in a spiral wringing motion squeezes and swings the blood into the aorta and pulmonary arteries. The electrical impulse that stimulates contraction is initiated in the SA node in the right atrium. For the contraction to begin at the apex, it is essential that the electrical impulse reach the apex of the ventricles before it can stimulate contraction of the myocardium at the base of the heart. This is accomplished by the specialized rapid ventricular conduction system extending from the AV node as the common His bundle. The His bundle branches into right and left bundles and these bundles send smaller branches throughout the ventricular myocardium. The conduction system is comprised of bundles of specialized large diameter muscle fibers located in the subendocardium throughout the ventricles. These are called Purkinje Fibers. Propagation of the impulse proceeds from the SA node through the atrium at about 0.1 to 1 m/sec and is greatly slowed while passing through the AV node to 0.01-0.05 m/sec. This is referred to as the AV delay. The impulse conduction in the ventricular system is very fast with a velocity of 2-4 m/sec in the His-Purkinje system versus 0.3-1.0 m/sec for the ventricular myocardium. The appearance of mature Apex-to-base conduction parallels the anatomical development of the His-Purkinje system.
A defining characteristic of embryonic cardiac muscle is that it can beat without any external stimulation and that the cells with the fastest intrinsic rate set the beat rate of the rest. The myocytes of the SA node have the most rapid heartbeat rate and are thus the pace maker. The mature node is distinguished by a rich network of nerve terminals representing both the sympathetic and parasympathetic divisions of the autonomic nervous system. The major function of the AV node is to provide for a delay as an electrical impulse is conducted from the atrial to the ventricular myocardium. The AV or His bundle originates at the posterior right atrial wall near the atrial septum and atrioventricular junction. It passes over the upper, fibrous margin of the ventricular septum and bifurcates near the base of the aorta into right and left bundle branches.
In early development the primitive heart tube is composed of uniform small myocytes. There is no morphologically distinct region of conducting cells. Nevertheless, conduction of the contractile impulse begins with the primary pace maker region located in the primitive atrium and presumably in the region of the future SA node. The primitive pace maker comprises the fastest beating myocytes and the conduction impulse spreads sequentially and uniformly from this group of cells towards the primitive ventricle and outflow tract. One characteristic that distinguishes slow conducting from fast conducting myocardium lies in the potential for intercellular communication in these two types of myocardium. Subsequently, the SA and AV nodes develop from the slow conducting myocardium of the inflow tract and atrioventricular canal. The His bundle and bundle branches develop from the fast conducting ventricular myocardium.
The primary heart tube is composed of slow conducting myocardium or primary myocardium. This is distinguished from working or fully differentiated chamber myocardium. The rapidly conducting “working” myocardium is thought by some investigators to represent more advanced myocardial differentiation. The slowly conducting myocardial zones appear to be essential for the function of the embryonic heart because alternating segments of slow and fast conduction are necessary for consecutive contraction of the tubular heart. The prolonged peristaltic contraction in the atrioventricular canal and outflow tract provides a one-way sphincteric function prior to the development of mature valves. Sequential activation of the atria and ventricles with an AV delay is reflected in the development of an adult-type electrocardiogram. The fast conduction system, represented by the ventricular conduction system because it develops from ventricular myocardium, includes the AV bundle, bundle branches, and Purkinje fibers, and these are the last to differentiate.
Innervation of the Developing Heart
The heart has both afferent and efferent innervation. The motor innervation is part of the autonomic nervous system which also provides motor information to all the viscera. Because of the integration of signals that is now recognized in the autonomic ganglia, there has been a tendency to include the sensory nerves as part of the ANS. I would prefer to maintain a separation and have the sensory innervation be considered as separate from the autonomic motor innervation. Sensory innervation of the heart is provided by neurons in the dorsal root ganglia. These cells bring sensory information from the heart to the CNS where they form reflex loops with motor neurons that effect changes in heart rate and/or the force of contraction. All motor autonomic output from the CNS is via pre-ganglionic to post-ganglionic neural relay. The pre-ganglionic neurons are located in the brain stem or spinal cord. They synapse on post-ganglionic neurons located in the periphery. The location of the pre-ganglionic neurons in the CNS and the post-ganglionic peripheral ganglia identify whether they constitute the PNS or SNS division of the autonomic nervous system.
In the PNS, the pre-ganglionic neurons are located in the brain stem. Their axons travel via the vagus nerve to the post-ganglionic neurons located in the cardiac ganglia that are in or around the heart. Some of these neurons around the heart have been labeled mirror neurons and are related to the literature on empathy and compassion. The sympathetic neurons are located in the intermediolateral cell column in the thoracic spinal cord and connect with the post-ganglionic neurons located in the lower cervical and upper thoracic sympathetic chain next to the vertebral column. All of the pre-ganglionic neurons of both the PNS and SNS use acetylcholine as their major neurotransmitter. Post ganglionic neurons use different transmitters. The PNS post ganglionic neurons use acetylcholine and are called cholinergic. These neurons exert tonic control over heart rate to slow it. The sympathetic post-ganglionic neurons use norepinephrine, a catecholamine, and are called adrenogergic. These neurons increase heart rate and force a contraction.
The development of this complex system of cardiac control is largely unexplored. What is known is first, all of the ANS post-ganglionic neurons derive from the neural crest; second, innervation of the heart is slow to develop and mature. In most animals, it is not fully functional until well after birth. This is especially true of a human infant whose autonomic nervous system goes through enormous growth in the first two years after birth and periodic growth spurts through the decade of the 20s. The onset of PNS activity precedes that of sympathetic activity in all species examined thus far. As a consequence, PNS control becomes functional and plays a role in cardiac function early than the SNS. However, it has been discovered that the heart itself synthesizes catecholamines and is susceptible to control by circulating catecholamines coming through the placental barrier from the mother’s blood. This may be a substitute for the late appearing SNS innervation.
All pre-ganglionic neurons form during early embryonic life. No new vagal motor neurons are added during fetal or post-natal development. The pre-ganglionic neurons connect with their post-ganglionic relays in the cardiac ganglia located on the surface of the heart or in nerve plexuses near the heart. The cardiac ganglia are widely distributed. They surround the SA node and the roots of the vena cavae and pulmonary veins. They are scattered on the posterior atrial wall, in the interatrial septum near the AV node and in the atrial appendages. The cardiac ganglia are associated with the origins of both the right and left coronary arteries in the human. The majority of atrial ganglia are situated in the sub-epicardial connective tissue. Two independent sets of ganglia selectively control SA rate and AV conduction. Cardiac rate is controlled by neurons located in the ganglia near the SA node while conduction is controlled by a separate rate of ganglia located at the junction of the inferior vena cava and the left atrium. Ventricular innervation is much sparser and arises from ganglia located in the plexus surrounding the roots of the aorta and pulmonary trunk. The structural integrity of the ganglia and their constituent neurons is dependent on constant stimulation from the vagus nerve. Cardiac ganglia serve as integrators of convergent neuronal activity rather than simple relays. Despite the morphological and biochemical similarities found among the cardiac ganglia in various locations in the heart, there are clear differences in the functionality among these ganglia.
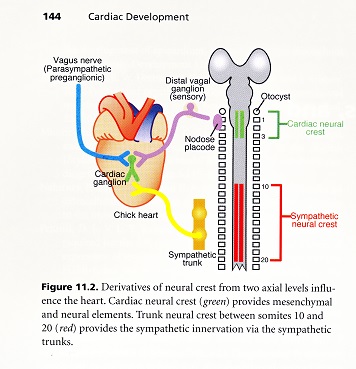
All of the post-ganglionic autonomic neurons are derived from the neural crest. Those that populate the cardiac ganglia originate from the cardiac neural crest which is located between the otic placodes and somite 4. The cardiac crest also provides ectomesenchymal cells that are necessary for septation of the outflow tract of the heart. During the first stages of PNS development, the cells that become principle neurons of the cardiac ganglia migrate from their origin. They carry information regarding their neuraxial level of origin which may be important in establishing communication with central nuclei. These cells are then contacted by pre-ganglionic fibers and a decision of which neurotransmitter to synthesize is made based on the peripheral structure innervated. In human development, cardiac ganglia have been seen from 7 weeks of gestation which is well before complete development of septation.
Growth of sympathetic nerves is mostly along blood vessels, suggesting that blood vessels regulate guidance of the nerves. The adrenal medulla consists of a large group of cells that lack neuronal processes as well as cells with typical neuronal morphology. In humans these two major cell types can be distinguished in the developing adrenal medulla as large cells with pale nuclei and ill defined cytoplasm. Both groups are present from the end of the embryonic period forward.
The level of autonomic innervation in the human at birth has been recently documented and has been found to be very similar to the adult pattern in density. Sympathetic fibers are found throughout the atrial and ventricular myocardium. PNS fibers are found throughout the atria but less so in the ventricles. Formation of morphological and functional control by ANS innervation occurs in the human heart well after the appearance of the reactivity to autonomic transmitters. It is seen that the embryo, fetus and infant goes through phases of sensitization, first to PNS agonists and then SNS agonists. For example, sympathetic tone becomes the dominant factor in heart development at the beginning of the third trimester, whereas parasympathetic tone is more dominant in the second trimester.